VERVANDIER-FASSEUR Dominique Voir la fiche profil en français 

- VERVANDIER-FASSEUR Dominique
- Statut : Associate Professor
- Team : OCS
- Function : Researchers
- Tags : Bio(in)organic chemistry, Chemical biology, Green chemistry, Synthetic chemistry
- ORCID :0000-0002-2998-5873
- Address :
ICMUB Institut de Chimie Moléculaire de l'Université de Bourgogne
Bât. MIRANDE - Aille B - Bureau B-323
9 Avenue Alain Savary
21000 Dijon – France - Tél : (+33) 380 399 036
- dominique.vervandier-fasseur@u-bourgogne.fr
Current Topic research: Synthesis and study of biological activities of trans-resveratrol derivatives
Background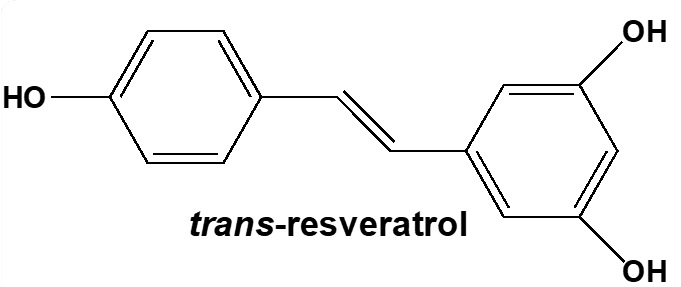
- The presence of trans-resveratrol in our daily diet is well-known. Although this natural polyphenol offers a broad range of biological activities, we focus on the synthesis of trans-resveratrol derivatives in the aim to better target and to improve some of these biological properties.
Results – Three series of 4-hydroxystilbenes were prepared and their biological activities were evaluated towards cancer colon cells SW480, hepatic tumor cells (HepG2) and two pathogen agents grapevine Plasmora viticola and Botrytis cinerea.
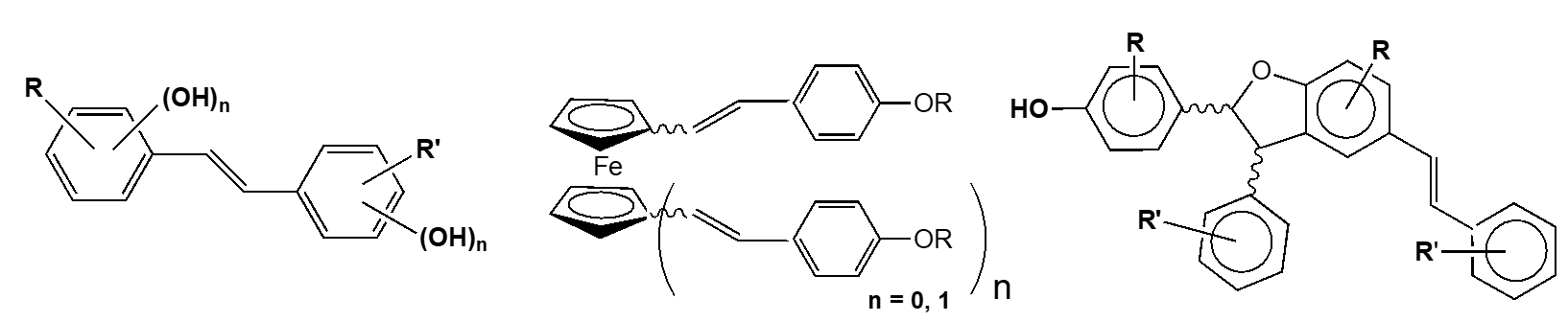
In each series, several trans-resveratrol derivatives display a higher activity than the parent molecule towards tumor cells SW480 and HepG2 as well as pathogen agents grapevine
Prospect – The promising results of these biological assays prompt us to develop the synthesis of new stilbenes and to diversify their applications. Four major projects are planned:
- Improvment of Wittig reaction conditions respecting the best principles of Green Chemistry to obtain easier a broad library of compounds and therefore, to expand biological tests.
- Development of stilbenes bearing a ferrocenyl moiety in the aim to enhance therapeutic activities of these stilbenes. Study by electrochemical way of their behavior in cellular environment for a best understanding of implied biochemical mechanisms.
- Grafting modified stilbenes on metallic and mesoporous nanomaterials for medicinal and theranostic applications.
- 4) Synthesis and study of antioxidant activities of hybrid molecules resveratrol-fatty acid
Collaborations –University of Burgundy: Prof. Norbert LATRUFFE & Dr. Gérard LIZARD – Lab Bio-PeroxIL; Prof. Marielle ADRIAN – AGROSUP-INRA uB-Agroécologie; Prof. Nadine MILLOT - ICB UMR CNRS 6303; Pr. Dominique DELMAS - INSERM UMR 866 ; National collaboration : Dr. Olivier BURIEZ - ENSCP Paris Tech; International collaboration :Prof. Corrado TRINGALI - University of Catania – Italie
F. Mazué, D. Colin, J. Gobbo, M. Wegner, A. Rescifina, C. Spatafora, D. Fasseur, D. Delmas, P. Meunier, C. Tringali, N. Latruffe. Structural determinants of resveratrol for cell proliferation inhibition potency: Experimental and docking studies of new analogs. Eur. J. Med. Chem., , 2010, 45,2972-80. doi : 10.1016/j.ejmech.2012.03.024
N. Latruffe, D. Delmas, G. Lizard, C. Tringali, C. Spatafora, D. Vervandier-Fasseur, P. Meunier. Resveratrol against pathologies: from diet prevention to alternative chemotherapies with new structural analogues.Invited chapter to the book entitled « Bioactive Compounds from Natural Sources » - 2nd Edition: Natural Products as Lead Compounds in Drug Discovery, Corrado Tringali Ed., Taylor & Francis Group, Boca Raton, Florida, 2011, 339-379
D. Vervandier-Fasseur, M. Chalal, P. Meunier. Procédé de préparation du trans-resvératrol et de ses analogues. Brevet Français, 11 56293, 2011
M. Chalal, D. Vervandier-Fasseur, P. Meunier, H. Cattey, J.-C. Hierso. Syntheses of polyfunctionnalized resveratrol derivatives using Wittig and Heck protocols. Tetrahedron, 2012, 68, 3899-907. doi : 10.1016/j.tet.2012.03.025
V. M. Bhusainahalli, C. Spatafora, M. Chalal, D. Vervandier-Fasseur, P. Meunier, N. Latruffe, C. Tringali. Resveratrol-related dehydrodimers : Laccase-mediated biomimetic synthesis and antiproliferative activity. Eur. J. Org. Chem,2012,5217-24. doi : 10.1002/ejoc.201200664
M. Chalal, A. Klinguer, A. Echairi, P. Meunier, D. Vervandier-Fasseur, M. Adrian. Antimicrobial activity of resveratrol analogues. Molecules, 2014, 19, 7679-88. doi : 10.3390/molecules19067679
M. Chalal, D. Delmas, P. Meunier, N. Latruffe, D. Vervandier-Fasseur. Inhibition of cancer cells derived cell lines proliferation by synthesized hydroxylated stilbenes and new ferrocenyl-stilbene analogs. Comparison with resveratrol. Molecules, 2014, 19,7850-7868. doi :10.3390/molecules19067850
S. Di Mocco, C. Spatafora, N. Cardullo, R. Riccio, K. Fischer, C. Pergola, A. Koeberle, O. Werz M. Chalal, D. Vervandier-Fasseur, C. Tringali, G. Bifulco. 2,3-Dihydrobenzofuran privileged structures as new bioinspired lead compounds for the design of mPGES-1 inhibitors. Bioorg. Med. Chem., 2016, 24,820-6. doi : 10.1016/j.bmc.2016.01.002
A. Namsi, T. Nury , H. Hamdouni, A. Yammine, A. Vejux, D. Vervandier-Fasseur, N. Latruffe, O. Masmoudi-Kouki, G. Lizard. Induction of neuronal differentiation of murine N2a cells by two polyphenols present in the Mediterranean diet mimicking neurotrophins activities: resveratrol and apigenin. Diseases, 2018, 6, 67. doi: 10.3390/diseases6030067
Prevention of 7-ketocholesterol-induced side effects by natural compounds. F. Brahmi, A. Vejux, R. Sghaier, A. Zarrouk, T. Nury, W. Meddeb, L. Rezi, K. Sassi, A. Yammine, I. Badreddin, D. Vervandier-Fasseur, L. Boulekbache-Makhlouf, B. Nasser,G. Lizard. Critical Rev. Food Sci. Nutr. 2018, 1-48.
Syntheses of polyfunctionalized resveratrol derivatives with potential antitumoral properties using Wittig and Heck protocols. Workshop NutriOx, Saarbrücken - Germany, September 2012
Syntheses of polyfunctionalized resveratrol derivatives with potential antitumoral properties using Wittig and Heck protocols. ASMOS VIII Dijon – France, November 2012
Syntheses of polyfunctionalized resveratrol derivatives with potential antitumoral properties Journée SCF Université-Industrie – Besançon - France – March 2013
Antitumoral and Antimicrobial Activities of Resveratrol Derivatives. WorkshopNutriOx – Metz – France, October 2014
Antitumoral and Antimicrobial Activities of Synthetic trans-Resveratrol Derivatives. Journées Méditerranéennes “Vin et Nutrition” – Hyères-les-Palmiers – France, March 2016
Antitumoral and Antimicrobial Activities of Synthetic trans-Resveratrol Derivatives. International Drug Discovery Science and Technology (IDDST) – Osaka - Japan, July 2017